pH-Based Strategies for an Efficient Addition of H2O2 During Ozonation to Improve the Mineralisation of Two Contaminants with Different Degradation Resistances |
| |
| Authors: | Ana de Luis José Ignacio Lombraña |
| |
| Affiliation: | 1.Department of Chemical Engineering and Environment, Faculty of Engineering in Bilbao,University of the Basque Country,Bilbao,Spain;2.Department of Chemical Engineering, Faculty of Science and Technology,University of the Basque Country,Leioa,Spain |
| |
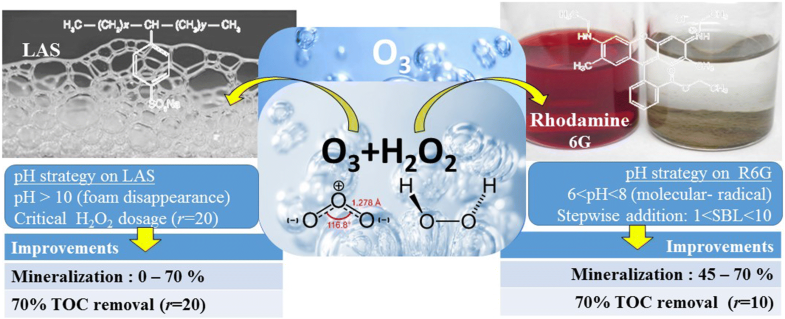
| Abstract: | Ozonation is an efficient process for the primary degradation of most substrates but not for their mineralisation. In this work, the ozonation enhanced with the addition of H2O2 was studied for two substrates with very different oxidation resistances: the dye rhodamine 6G (R6G) and the surfactant linear alkylbenzene sulfonate (LAS). With O3 only, the primary degradation of R6G was completed in less than 10 min but its TOC removal only reached 45% in 1 h. By adding H2O2, TOC removal was increased to 70% with a molar ratio (mol H2O2/mol substrate) of 10. The analysis of pH decrease served to define the specific basicity loss (SBL). The optimum conditions for the R6G mineralisation were found to be associated with a SBL value between 1 and 10 ((min/g)/L)?1, through an adequate addition of H2O2. Moreover, in the case of LAS, the addition of H2O2 for a greater efficiency should occur after the foaming period, above all formed at acid pH. LAS degradation was also considerably improved, and the optimum for primary degradation achieved in 10 min with a TOC removal of over 65% with a molar ratio (mol H2O2/mol substrate) of 20. |
| |
| Keywords: | |
| 本文献已被 SpringerLink 等数据库收录! |
|